Purpose: This exercise was designed to:
- Get students to understand reversal potentials (ERev) and how it relates to equilibrium potentials.
- Get students to understand the dissociation between excitation/inhibition and depolarization/hyperpolarization.
- Get students to understand that it is the receptor properties, such as reversal potential, that determines the end effect, not the neurotransmitter.
- Get students to understand how current relates to voltage changes at the synapse.
Structure of exercise:
- The exercise is structured into 2 parts. Parts A & B are for general contemplation of synaptic function. Parts 1-5 are assigned to a group specifically to be prepared to answer. Students are free to solve other groups problems if they have additional time while waiting.
(A) What are the differences between an electrical synapse vs. chemical?
(B) What are the differences between a metabotropic vs. an ionotropic receptor. Think of an ACh receptor that is metabotropic and an ACh receptor that is ionotropic and where they are located.
1. If you apply glutamate to a receptor of unknown action, is the effect likely excitatory or inhibitory? Vm = -60 mV. What additional pieces of information would you need to be absolutely certain whether it is excitatory or inhibitory?
Purpose: This question is designed to make them think about neurotransmitter and likely associated effect, but also, to not always make that blanket assumption of because it is this neurotransmitter, the end effect is “x”.
Answer: It is glutamate, so it is likely an excitatory effect on that receptor. However, to be absolutely certain, one would need to know what ERev is in relation to the threshold value. So, you need to know ERev & threshold. If ERev < threshold, inhibitory. If ERev > threshold, excitatory.
Assume the below for the following question if value not asked for:
Vm = -60 mV Erev = 40 mV ECl = -80 mV
ENa = +40 mV Ek = -70 mV Threshold = -20 mV
2. If the ERev of the channel is determined by Na+ and K+, and its conductivity for Na+ and K+ are equal, i.e., gna = gk for that channel, can you determine ERev? Why or why not? What is ERev for this channel?
Purpose: This question is designed to get students to understand that ERev is governed by the equilibrium potentials of the ions which it is permeable/conductive to.
Answer: ERev is determined by Na+ and K+ in this example. If the conductances are equal, then ERev is in the middle of ENa & EK . If you do the calculations, based on the equilibrium potentials provided above… ENa & EK = 40 + – 70 / 2= -15 mV.
3. An unknown receptor has been identified which binds GABA. It has an Erev of +40 mV. If I apply GABA to this unknown receptor, is the post-synaptic potential (PSP) excitatory or inhibitory? Is the effect a depolarization or a hyperpolarization? Is the current inward or outward? Be ready to explain.
Purpose: This question is designed to get students to dissociate the neurotransmitter from the receptor function. GABA is typically associated with hyperpolarization and inhibition, but it is not always the case. The students need to test their understanding of what governs excitation/inhibition and depolarization/hyperpolarization.
Answer:
First, calculate current. Using ERev is +40 mV, we can calculate driving force. Vm is given as -60 mV. I = (g)(Vm – ERev) = -60 mV – +40 mV = -100. Remember, we make the assumption that g is 1 (perfectly conductive for that channel), so we can calculate out the current direction and whether its positive/negative. If driving force is -100, this means that the current is negative, and thus inward. Once again, to reiterate the point, we are discussing conventional current flow, which is defined by the flow of + ions.
First, calculate current. Using ERev is +40 mV, we can calculate driving force. Vm is given as -60 mV. I = (g)(Vm – ERev) = -60 mV – +40 mV = -100. Remember, we make the assumption that g is 1 (perfectly conductive for that channel), so we can calculate out the current direction and whether its positive/negative. If driving force is -100, this means that the current is negative, and thus inward. Once again, to reiterate the point, we are discussing conventional current flow, which is defined by the flow of + ions.
Second, use current to determine if it is depolarizing or hyperpolarizing. If current is negative and inward, this is making the inside of the neuron more positive, as the GABA bound channel is trying to bring the neuron to its ERev at +40 mV. While the channel is open due to GABA binding, the neuron is pulled towards ERev.
Third, use threshold and compare it to ERev to determine if it is excitatory or inhibitory. If ERev < threshold, it is inhibitory. If ERev > threshold, it is excitatory. In our case, our ERev is +40 mV, and threshold is -20 mV. ERev > threshold, thus excitatory.
4. If the ERev is instead -30 mV, and I apply GABA, is the post-synaptic potential (PSP) excitatory or inhibitory? Is the current inward or outward? Is the effect a depolarization or a hyperpolarization? Be ready to explain.
Purpose: The goal of this is to get students to dissociate depolarization with excitation… and make them understand instances in which it may be inhibitory.
Answer:
First, calculate current. Using Erev at -30 mV, we calculate driving force. Vm is -60 mV as a given. -60 – – 30 = -60 + 30 = -30 mV. Current is negative, thus inward.
Second, determine depolarization/hyperpolarization. Because it is inward and negative current, it is a depolarization.
Third, determine excitation/inhibition. Once again, use ERev < threshold = inhibition, ERev > threshold = excitation. In this case, ERev is -30 mV, threshold is -20 mV. In this case, ERev < threshold, thus inhibitory. Do not get confused by depolarization/hyperpolarization and make the mistake of absolute association with depolarization = excitation / hyperpolarization = inhibition. There are instances, such as in this example, where depolarization leads to inhibition, because the channel prevents the neuron from ever getting to threshold… If the membrane potential ever gets more positive than ERev, the current will turn positive, with an outward flow of positive ions to try to bring the membrane potential back to ERev. For example, if the membrane potential somehow gets to -25 mV, -25 mV – – 30 = +5… thus current would be outward, positive ions flowing out, making the cell more negative again… and most importantly, pulling it AWAY from threshold.
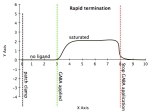
5. If I patch clamp the channel at +40 mV, then apply GABA to that channel 3 seconds after the patch clamp for an additional 5 seconds while under patch clamp (seconds time scale), what would the current trace look like? Assume Erev is the same as in #5. Draw 10 seconds worth of recording, starting immediately after the patch clamp.
Purpose: The purpose of this question is 2-fold. First, to get them to understand that without ligand binding, the function of the receptor/channel is non-existent,because it is not open. Secondly, to get them to understand the relationship between current and voltage/channels.
Answer:
Calculate driving force. Because we patch clamp at +40 mV (note, patchclamp means we’re typically looking at only a patch of tissue with hopefully only the receptor channels we are interested in), our membrane potential is at +40 mV. +40 mV – -30 mV = +10. This means current is outward and positive. In terms of drawing the trace:
Calculate driving force. Because we patch clamp at +40 mV (note, patchclamp means we’re typically looking at only a patch of tissue with hopefully only the receptor channels we are interested in), our membrane potential is at +40 mV. +40 mV – -30 mV = +10. This means current is outward and positive. In terms of drawing the trace:
seconds 0-3, while we have patch clamped the neuron at +40 mV, there is no current. This is because it is ligand dependent. If there is no ligand, there is no open channel, and thus no current.
seconds 3-8, there will be some outward current. The exact slope doesn’t matter, as long as you understand it rises. It depends on the receptor action and how much we flood the synapse with neurotransmitter for determining the slope of the current (so, it could even be what looks like an immediate vertical rise rather than a slope). If it was ionotropic, the rise would probably be much steeper. If its metabotropic, less so. However, it depends on the timescale we’re looking at. If we’re looking at seconds, its probably pretty steep. If we’re looking at milliseconds, we could probably see the difference between the metabotropic vs. ionotropic.
seconds 8-10, there will either be some drop off that could be steep or a slower plateau. Depends on the molecule, and whether there are enzymes to chew up the neurotransmitter. There could also be transporters to re-uptake the neurotransmitter. A final point is that it also depends on the time scale we’re looking at.
Below, I’ve traced 2 versions. One in which termination is fairly slow. The other in which termination is a faster. These are conceptual images. If termination is slower, its likely that the synapse has less enzymatic breakdown and less transporter reuptake. If the termination is rapid, its likely the synapse has (1) alot of enzymes breaking down the neurotransmitter and/or (2) alot of reuptake.
- Slower termination, if there is less reuptake and breakdown of neurotransmitter
- Faster termination, if there is more reuptake and breakdown of neurotransmitter